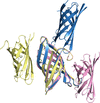issue contents
April 2024 issue
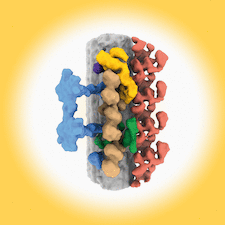
Cover illustration: The use of electron microscopy to visualize ciliary axonemes [Walton et al. (2024), Acta Cryst. D80, 220–231]. Cryo-ET and subtomogram averaging of ciliary axonemes has revealed the architecture of the 96 nm repeat unit of the doublet microtubules, providing the location and shape of many axonemal complexes including outer dynein arms, inner dynein arms, radial spokes, the nexin–dynein regulatory complex and the tether/tetherhead complex.
CCPEM
Open  access
access
 access
accessThis review describes how electron-microscopy methods have helped to reveal the structure of the ciliary axoneme.
Open  access
access
 access
accessThis paper presents recent technical improvements to the VitroJet and the benefits that it brings to the cryo-EM workflow, as well as a wide variety of case studies. This illustrates the advancement of the VitroJet into an instrument that enables accurate control and reproducibility, demonstrating its suitability for time-efficient cryo-EM structure determination.
Open  access
access
 access
accessTo simplify the cryo-electron tomography workflow and make this technique more accessible to all researchers, the Tomo Live software has been developed, which performs on-the-fly reconstruction of tilt series, enabling real-time data-quality monitoring, curation and export.
Open  access
access
 access
accessEMinsight is a Python-based tool for systematically mining metadata from single-particle analysis cryoEM experiments. The capture and analysis of metadata facilitates the assessment of instrument performance, provides concise reporting of experiment performance and sample quality by analysing preprocessing results, and gathers metadata for deposition. It is envisaged that this approach will benefit the microscope operator, facility managers, database developers and users.
research papers
Open  access
access
 access
accessThe structure determination of a small protein of unknown function by molecular replacement using a search model predicted by new machine-learning methods is reported. Notably, the approach was successful using electron diffraction data collected from a protein microcrystal, highlighting a potentially important new route for structure determination.
Open  access
access
 access
accessA sample- and time-efficient method to obtain serial crystallography data from batch-grown microcrystals dispensed as drops on a 96-well crystallization plate is described. This offers a versatile method to obtain low-dose room-temperature structures and guide the optimization of microcrystallization for synchrotron and XFEL serial crystallography experiments.
Open  access
access
 access
accessThe crystal structures of europium(III)- and gadolinium(III)-bound phosphotriesterase are presented along with esterase activity data for the lanthanide-bound enzymes.

 journal menu
journal menu